日本住友化学 SUMICHIRAL色谱柱
日本住友化学代理
日本住友化学官网
欢迎访问日本住友化学SUMICHIRAL官网或者咨询我们获取更多相关产品信息。
SUMICHIRAL OA columns are high-performance chiral columns for
enantiomer separation by HPLC. On SUMICHIRAL OA columns direct
separation of various enantiomers can be realized effectively.
Enantiomeric separation is achieved from the various diastereomeric
interactions such as hydrogen bonding, charge transfer and host-guest
interactions, etc. SUMICHIRAL OA columns are very useful for the
accurate determination of the optical purity and for the preparation of
pure enantiomers of biologically active compounds such as
pharmaceuticals, pesticides, and perfumes.
■ Improved Pirkle Type
■ Ligand exchange Type
■ Host-guest Type
Improved Pirkle Type 1 Improved Pirkle Type 1
■Amide Type : Asymmetric carbon atoms are bonded directly with CONH group
OA-2000 series have a 3,5-dinitrobenzoyl group as theπ-acid and may interact with the solute
molecule by charge transfer, hydrogen bonding, etc. The enantiomers of aromatic compounds, esters,
carboxylic acids and alcohols may be directly separated on OA-2000 series. OA-2000 is especially
effective for pyrethroidal esters, OA-2500 for carboxylic acids such as profen-drugs.
■Urea Type : Asymmetric carbon atoms are bonded directly with NHCONH group
OA-3000 series have 3,5-dinitrophenylurea group as theπ-acid and, in the reverse phase mode,
promote chiral discrimination by charge transfer, hydrogen bonding, etc. In general OA-3000 series are
effective for carboxylic acids, and especially for acetyl- and urethane-amino acids, dansylamino acids.
OA-3300 offers good direct separation for profen-drugs, acetyl-amino acids, BOC-amino acids and
benzyl-amino acids.
Main columns : SUMICHIRAL OA-2500, SUMICHIRAL OA-3300
Special Merits of SUMICHIRAL OA
The large number of theoretical plates of the columns offer high
resolution.
The packing materials have chemical stability and the columns have
long life.
The enantiomeric stationary phases give the inverse elution orders
and so accurate determination of the optical purity and efficient
preparation of the enantiomer are attained.
Improved Pirkle Type 2 Improved Pirkle Type 2
Two chiral centers at amine and amino acid are bonded with NHCONH group
OA-4000 series have a naphthyl group as theπ-base, and two chiral centers at amine and amino
acid group. By charge transfer, hydrogen bonding, etc., chiral discrimination is acheived, and a wide
variety of compounds such as pharmaceuticals of amine and amino alcohols, alcohols, esters and
amides can be directly resolved in the normal phase mode. Amide and urethane derivatives of amines,
alcohols, etc. can be resolved effectively
Ligand exchange Type Ligand exchange Type
■The chiral components are coated hydrophobically on ODS
OA-5000 and 6000 series offer chiral discrimination by ligand exchange interaction in the
reversed phase mode. The chiral ligands such as penicillamine (OA-5000) or tartaric acid
derivatives (OA-6000 series) are coated on ODS silica, though the volume of organic solvents
added to the mobile phase is limited. Mobile phases including Cu++ ions are used in these
columns. They are effective for direct enantiomer separation of not only amino acids, hydroxy acids
but also copper-chelate forming compounds such as amino alcohols, diamines, dicarboxylic acids,
aminolactames and dipeptides. Especially OA-5000 can be applied for extremely wide range, while
OA-6100 is effective for β-amino acids,β-hydroxy acids and hydrophilic amino acids.
Main columns : SUMICHIRAL OA-5000, SUMICHIRAL OA-6100
Host-Guest Type
■Cyclodextrin bonded chiral stationary phase with novel spacer
OA-7000 is a novel chiral stationary phase with β-cyclodextrin bonded to the silica gel via new type of
spacer. A large number of racemates, including ketones, amines, and amino acid derivatives can be
separated under reversed phase conditions.
(1) Sharp peaks and high theoretical plate numbers are obtained.
Improved peak shape is due to the effect of hydrophilic spacer moiety which prevents secondary
interactions between the silica gel and the sample molecules.
(2) Popular reversed phase conditions can be used.
■Novel Chiral Stationary phase bonded with crown ether
OA-8000 is a novel chiral stationary phase bonded with chiral crown ether to aminopropyl silica gel. This
is very effective for enantiomer separations of amines, aminoalcohols and amino acids, especially for
hydrophobic amines.
(1) Stationary phase is covalent bond type and very stable.
(2) Both reversed and normal phases can be used.
(3) Sharp peaks and high theoretical plate numbers are obtained
| Standard type | Inverted type | Mode** | ||
| SUMICHIRAL | Chiral component | SUMICHIRAL | ||
| OA-2000 | (R)-phenylglycine | OA-2000S | NP | |
| ☆ OA-2500 | (R)-1-naphthylglycine | OA-2500S | RP | |
| OA-3100 | (S)-valine | OA-3100R | NP,RP | |
| OA-3200 | (S)-tert-leucine | OA-3200R | NP,RP | |
| ☆ OA-3300 | (R)-phenylglycine | OA-3300S | NP,RP | |
| OA-4000 | (S)-valine (S)-1-(α-naphthyl)ethylamine | OA-4000R | NP | |
| OA-4100 | (S)-valine (R)-1-(α-naphthyl)ethylamine | OA-4100R | NP | |
| OA-4400 | (S)-proline (S)-1-(α-naphthyl)ethylamine | OA-4400R | NP | |
| OA-4500 | (S)-proline (R)-1-(α-naphthyl)ethylamine | OA-4500R | NP | |
| OA-4600 | (S)-tert-leucine (S)-1-(α-naphthyl)ethylamine | OA-4600R | NP | |
| ☆ OA-4700 | (S)-tert-leucine (R)-1-(α-naphthyl)ethylamine | OA-4700R | NP | |
| OA-4800 | (S)-indoline-2-carboxylic acid | * | NP | |
| (S)-1-(α-naphthyl)ethylamine | ||||
| ☆ OA-4900 | (S)-indoline-2-carboxylic acid | * | NP | |
| (R)-1-(α-naphthyl)ethylamine | ||||
| ☆ OA-5000 | (D)-penicillamine | OA-5000L | RP | |
| OA-6000 | (L)-tartaric acid (S)-1-(α-naphthyl)ethylamine | OA-6000R | RP | |
| OA-6100 | (L)-tartaric acid, (S)-valine | OA-6100R | RP | |
| (R)-1-(α-naphthyl)ethylamine | ||||
| ☆ OA-7000 | β-cyclodextrin with novel spacer | * | RP | |
| ☆ OA-8000 | chiral pseudo 18-crown-6 ether | * | NP,RP |
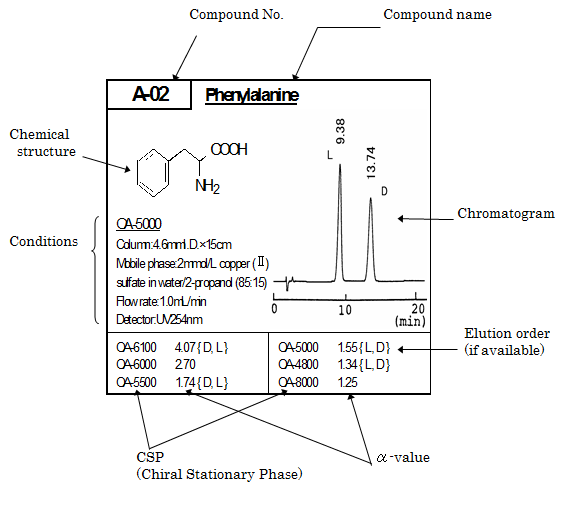
Notation on the SUMICHIRAL database
SUMICHIAL标签的表示法